Abstract
Cancer requires transcriptional misregulation to enable reprogramming into a stem cell-like state, as in many subtypes of acute myeloid leukemia (AML) through the maintenance of HOX genes. While most AML drivers of aberrant HOX transcription, such as MLL-rearranged or NUP98 fusion proteins, reside in the nucleus and have protein domains associated with gene regulation, the most common driver of adult AML, mutant Nucleophosmin 1 (NPM1c), lacks any evident connection to transcriptional regulation. Cytoplasmic NPM1, or NPM1c, results from the addition of a nuclear export sequence (NES) onto the essential nucleolar protein NPM1, leading to its strong relocalization into the cytoplasm. While we and others have previously implicated NPM1c in directly regulating HOX expression, the mechanism through which this cytoplasmic oncoprotein paradoxically drives nuclear activities remains unresolved.
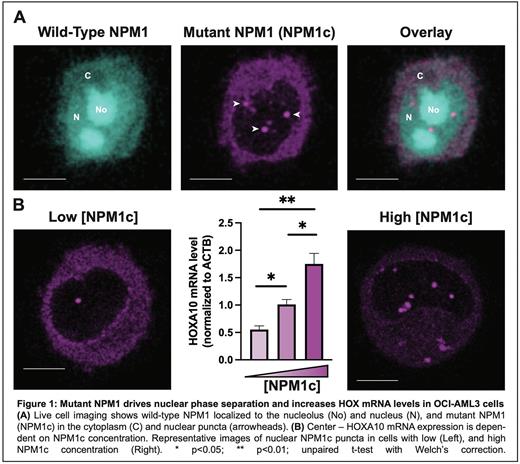
To investigate NPM1c dynamics and identify potential nuclear functions, we employed high-resolution microscopy in human AML cell lines. Consistent with expectations, NPM1c molecules overwhelmingly reside (>90%) in the cytoplasm with a negligible amount in the nucleoplasm and nucleoli. Strikingly, we consistently observed NPM1c localization to discrete nuclear puncta distinct from wild-type NPM1 (Figure 1A). Biophysical characterization revealed that these puncta require a critical concentration of NPM1c for their formation, and their size and number increased with higher concentration of NPM1c. These properties are defining features of phase transitions, allowing us to unequivocally designate the puncta as phase-separated condensates. Remarkably, NPM1c is enriched in nuclear condensates >100 fold higher than in the cytoplasm, and wild-type NPM1 is absent from the puncta. We next sought to determine whether NPM1c condensates could drive HOX transcription. To our surprise, we found that HOXA10 expression depended on NPM1c concentration, increasing as additional phase separation occurred, highlighting a novel role for NPM1c condensates in regulating HOX transcription (Figure 1B). Furthermore, extensive truncation and mutagenesis of NPM1c allowed us to identify critical domains required for phase separation, HOX expression, and maintenance of the stem-cell-like state. Misregulated HOX expression is pathognomonic of NPM1c-driven AML; therefore, these results definitively associate NPM1c condensates and the transcriptional program of AML.
To elucidate the mechanisms through which these condensates could drive HOX transcription, we sought to identify other factors that may play structural and functional roles. We found that XPO1, a known binding partner to NPM1c's NES, co-localizes with NPM1c condensates. Treatment with XPO1 inhibitors (Selinexor and Eltanexor) resulted in rapid relocalization of cytoplasmic NPM1c to the nucleus. However, disruption of NPM1c condensates was significantly delayed and occurred after extended treatments. These results are consistent with reports that prolonged XPO1 inhibition may enhance anti-leukemic activity and provide new insights into the suboptimal clinical performance of XPO1 inhibitors.
Furthermore, the delayed dissolution of NPM1c condensates following XPO1 inhibition suggested other factors are involved in condensate formation. Indeed, we identified the presence of multiple proteins previously implicated with the HOX transcription program, including NUP98 and MLL, within NPM1c condensates. This observation raises the possibility that NUP98 and MLL fusion proteins found in other HOXA-driven AML sub-types may also function through phase-separated condensates. To that end, we explore relevant fusion proteins found in hematological malignancies and assess their capacity for driving condensates that activate HOX transcription.
Altogether, our results suggest that nuclear phase separation, driven by NPM1c or other HOX-associated drivers, is a ubiquitous mechanism underlying AML, providing a solid foundation for developing new therapeutics targeting common vulnerabilities across AML subtypes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal